
Natural Rubber: Structure and Function
Beyond the Cis-1,4 structure – Some Reasons Why Synthetic Rubber May Never Replace Natural Rubber.
By D.J. Miller
Natural Rubber Consultant
In 1963 Karl Ziegler and Giulio Natta shared the Nobel Prize in Chemistry for the development, in the 1950’s, of their eponymous catalysts for the production of stereoregular polymers from propylene. Their catalyst, an organoaluminum compound coupled with a transition metal, led to the development of synthetic rubbers with a structure closely resembling natural rubber.1,2 At about the same time, researchers elsewhere developed alkyllithium catalysts which resulted in similar “synthetic natural” rubber structures.3 With those developments, the structure of natural rubber, thought only to be the provenance of nature’s enzymatic control, could be copied by man. But this did not lead to the displacement of natural rubber in industry. More than 50 years after the development of synthetic high cis polyisoprene and high cis polybutadiene, natural rubber still occupies an irreplaceable position in the rubber industry. Why has synthetic rubber not replaced natural rubber? It is because of the unique structure of natural rubber and the properties this structure confers to industrial products. This structure/property relationship will be investigated in this paper.
Natural Rubber Structure
The characterization of polymer materials is more complex than the characterization of simple organic molecules. It has become the norm to separate macrostructure from microstructure when discussing polymer structures. Macrostructure includes the average molecular weight and molecular weight distribution of the individual polymer molecules. Microstructure refers to the way individual monomer units are distributed along the chain and the geometry in which they are distributed.
Macro Considerations
Natural rubber is a polymer, a long, chain like molecule that contains repeating subunits. The term polymer comes from the Greek “poly” meaning many and “mer” meaning parts. The chemical name for natural rubber is polyisoprene. The monomer (meaning “one-part”) from which it is built is isoprene. It is worth mentioning here that, although natural rubber is built of repeating isoprene units, isoprene is not the starting monomer for the natural product. Natural rubber is the result of a series of biochemical reactions which start with isopentenyl pyrophosphate within the tree.4
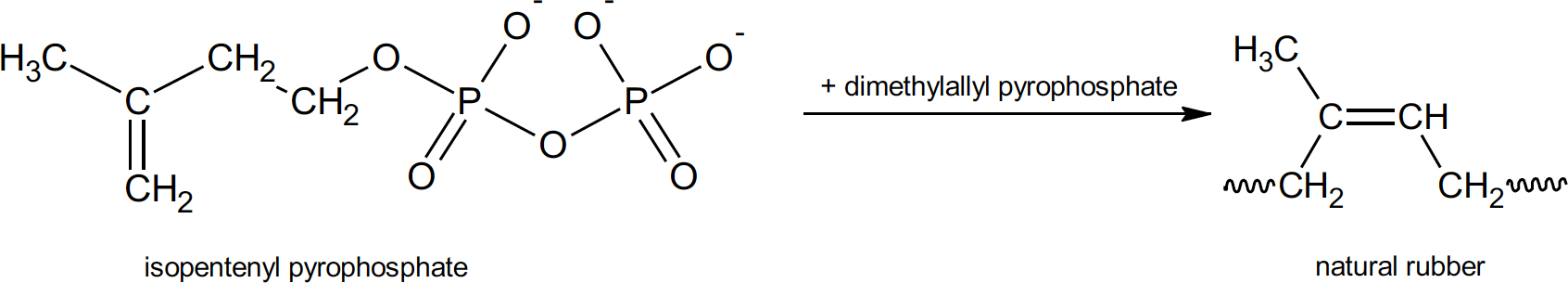
Biochemical production of natural rubber
One can make use of the analogy of a plate of tangled spaghetti representing a polymer mass. The individual spaghetti stands represent a single polymer chain. The long chain length allows for entanglement. The entanglement helps hold the mass together. When a forced is applied to the spaghetti, as in lifting it with a fork, the strands will tend to untangle and straighten out just as a polymer strand is uncoiled and rotations occur about single bonds in the polymer backbone. The individual spaghetti strands will also slide by each other and when the force is released the mass may not return to its original orientation just as polymer chains may not fully recover and may take a set. This movement of polymer chains past one another limited the use of natural rubber as previously mentioned. With the discovery of vulcanization, a structure could be formed with sulfur bonds linking individual polymer chains into a 3-dimensional network. Chains that could previously flow past one another under stress now had limited extensibility which allowed for the support of stress and a retraction upon the release of the stress. Our spaghetti analogy has just turned from unconnected spaghetti stands to a fishing net structure as in vulcanized rubber.
When considering the macrostructure of natural rubber, one must note the very high molecular weight of natural rubber. With a molecular weight range of from 3 x 104 to 1 x 107, it is several times more, at the high end, than most synthetic rubbers.5 This high molecular weight results in less chain ends and more entanglement than an equal weight of synthetic rubber. Since chain ends are a weak point at the molecular scale, because they do not transmit the strength of covalent bonds in the molecular chain, tensile strength is greater for higher molecular weight polymers like natural rubber.6 Another macrostructure property is the degree of branching of a polymer. That is, how many bulky polymer side groups may be attached to the polymer backbone. Branching affects the glass transition temperature (Tg) of a rubber, which is a stiffening that occurs when the temperature is lowered. It is the temperature at which the transition from a glassy state (hard, brittle) to a rubbery state occurs as the temperature of a glassy polymer is raised. This transition usually occurs over a range of temperatures, but is often quoted as the mid temperature of this range. This phenomenon is distinct from crystallization (discussed below) and can result from bulky side groups on the polymer chain, which are absent in natural rubber. The Tg of natural rubber is -72°C.
Microstructure
Polyisoprenes can feature four different isomers in its polymer chain. These are cis-1,4; trans- 1,4; 1,2; and 3,4. Isomers (meaning equal parts) contain the same number of atoms of each element but have a different arrangement of those atoms. The numbers in the isomer name refers to the particular carbon atom in each unit which are attached to adjacent units. So, in a 1,4 structure, carbon atoms 1 and 4 are joined in forming the chain. Cis means that the 1 and 4 carbon atoms are attached to the same side of the carbon-carbon double bond. Trans structure refers to the 1 and 4 carbon atoms attached to the opposite side of the double bond. It should be mentioned that these isomers are different because there is no rotation around a carbon-carbon double bond as there is with single bonds. Natural rubber consists almost entirely of the cis-1,4 structure and hence is, chemically, cis-1,4 polyisoprene. When the chain units in a polymer consists of the same isomer it is said to be stereoregular.
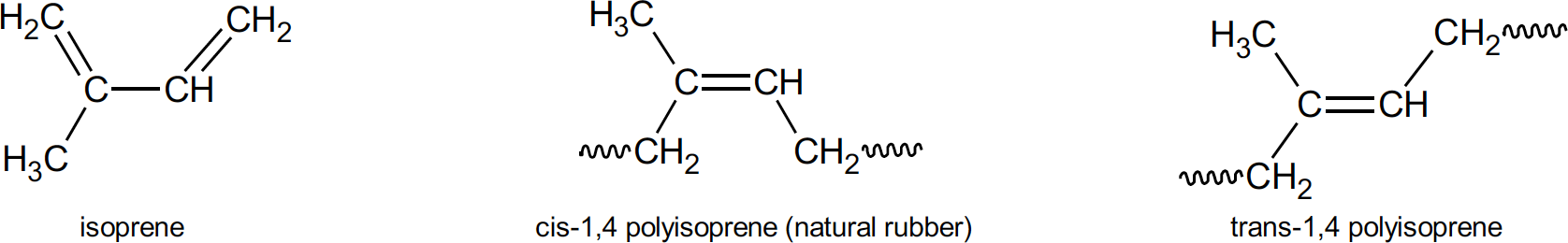

Isomers of polyisoprene
It is this stereoregularity, the nearly 100% cis-1,4 polyisoprene structure of natural rubber, that confers many of the desirable properties to the mechanical goods natural rubber is used to produce.7 Crystallinity of natural rubber is a characteristic provided by this microstructure. If the units of a polymer chain are in a regular enough spatial arrangement then interactions between these units from polar attraction, hydrogen bonding or functional groups, will form crystalline structures which stiffen the polymer. Natural rubber, because of its stereoregularity, will form crystallites upon storage and upon stretching. Although crystallization upon storage can cause some processing difficulties (it can be reversed upon heating), the reversible crystallization upon stretching, so called strain induced crystallization, caused by intermolecular forces in the polymer, provides many of the unique properties of natural rubber. Specifically, these are its excellent green strength (uncured rubber strength) and building tack, both of which are of prime importance in tire building. In the final product, strain induced crystallization results in excellent cut resistance, tear resistance, tensile strength and cut and crack growth resistance.8 Resilience, the result of natural rubber’s polymer network upon curing which allows for elasticity and flexibility, combined with crystallization induced toughness when stretched means less kinetic energy is lost as heat during repeated stress deformation. In truck and bus tires, and in passenger sidewalls, natural rubber is used extensively to provide this low heat build-up. Heat build-up is an undesirable but unavoidable consequence of tire deformation when rolling down the road. Energy losses are converted to heat which is not easily conducted away in a low thermal conductivity material such as rubber. In heavy duty truck tires shoulder temperatures (the area where the tread and sidewall meet) may be up to 100°C higher than other surface temperatures on the tire. Heat generation of this magnitude runs the risk of blow-out or other delamination processes related to crack growth.9 In another scenario, picture a truck backing slowly into a loading dock and accidently going over a curb at a shallow angle. Tremendous stresses are placed on the tread and sidewall of the truck tire as it backs up over the curb causing strain in the sidewall. The resulting strain induced crystallization stiffens and strengthens the rubber by forming crystallites which act, in effect, as a reinforcing filler. Tears and cuts could initiate in anything less than natural rubber under these conditions. In passenger tires, natural rubber is used in the carcass and sidewall areas. There, strain induced crystallization provides the building tack and ply adhesion properties required.
As mentioned earlier, in the mid 1950’s, two catalytic methods were developed which were then used to attempt production of “synthetic natural” rubber, i.e. stereospecific polyisoprene and polybutadiene. Both of these processes were developed in the U.S.A. The search for these processes may have been motivated by fears that further world conflict after World War II would again cut off supplies of natural rubber from the Far East and that the U.S. should not again be in a positon of dependence on foreign sources of natural rubber. The alkyllithium polymerization process yielded polyisoprene with a cis-1,4 content of approximately 94%. The Ziegler-Natta process yielded polyisoprene with a cis-1,4 content of approximately 96%.10 Although these two polymers, along with some high cis polybutadiene polymers with up to 99% cis content, exhibit strain induced crystallization, they still did not have the required rate and degree of crystallization to provide all of the properties of natural rubber in tire applications.
Other Structural Considerations
Obviously, there is more than just high cis content that provides the combination of rate and degree of crystallization exhibited by natural rubber. Besides the hydrocarbon portion, natural rubber contains approximately 6% non-rubber constituents; ~2.2% proteins, ~3.4% lipids (fatty acids), glycolipids and phospholipids, and ~0.4% carbohydrates.11 It has been found that deproteinized natural rubber still exhibits the crystallization effects and associated properties, so proteins are not contributing factors. Recently, through a model of cis-polyisoprene grafted with stearic acid, it has been shown that saturated fatty acids linked to the rubber chain induce crystallization, while mixed unsaturated fatty acids, which are present in natural rubber, act as a plasticizer and accelerate the crystallization rate. Then, through nuclear magnetic resonance (NMR) studies, the chain ends of natural rubber were found to contain two trans isoprene units and an oligopeptide at one end and a phospholipid terminal group at the other which matched the crystallinity effects of the stearic acid grafted polyisoprene model above.12,13 These polymer terminations may also allow natural rubber to act as a functionalized polymer resulting in branching at the chain ends.
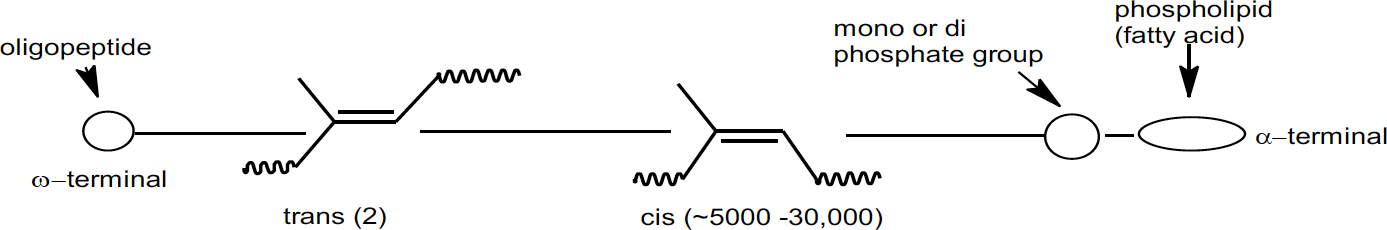
Schematic representation of natural rubber polymer chain
These results are leading researchers to conclude that, in addition to the nearly 100% cis-1,4 polyisoprene microstructure, the non-rubber constituents in natural rubber contribute significantly to the unmatched properties of natural rubber in industrial products such as tires.
Concluding Thoughts
In the years following the development of stereoregular “synthetic natural” rubber, it was widely thought that the natural rubber producing industry would suffer a slow demise. This has not come to pass. Although synthetic rubber is used extensively in blends with natural rubber, the complete replacement has not occurred. The reasons stem from the fact that the properties provided to industrial products are not duplicated by the mere copying of the microstructure of natural rubber. The biosynthetic pathways used by Hevea brasiliensis incorporates terminal groups to the polymer chain that have not been reproduced by synthetic means, and because of their complexity, may never be copied. Additionally, several other factors play into the equation. 1) The continual rise of oil prices, and the products derived therefrom, places limits on the economies of synthetic rubber replacing natural rubber. 2) The fact that natural rubber is produced from a renewable source – the rubber tree, and, 3) this source is, for the most part, environmentally friendly.14 Considering all of these factors, one may reasonably conclude that natural rubber is here to stay.
About our guest blogger: D.J. Miller is a natural rubber consultant based in the USA with over 40 years of experience in the tyre, natural rubber and natural latex industry. He was Senior Technical Service Engineer for Firestone for 14 years and was in research and development for 26 years for mostly one company that went through various name changes (Edmont, Ansell Edmont and then Ansell). We thank him for this report.
1. “The Nobel Prize in Chemistry 1963”, https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1963/.
2. Blackly, D.C., Synthetic Rubbers: Their Chemistry and Technology, Chapter 2, Applied Science Publishers, London, 1983.
3. Ibid.
4. Cornish, K., Blakeslee, J., Rubber Biosynthesis in Plants, http://lipidlibrary.aocs.org/Biochemistry/content.cfm?ItemNumber=40312.
5. Roberts, A.D., Editor, Natural Rubber Science and Technology, Malaysian Rubber Producers Research Association, 1988.
6. Sperling, L. H., Introduction to Physical Polymer Science, Chapter 1, John Wiley & Sons, 1986.
7. Morton, Maurice, Rubber Technology, Van Nostrand Reinhold Company, Second Edition, 1973.
8. Kent, James A., Editor, Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology, Eleventh Edition, Chapter 16, Springer, 2007.
9. Roberts, A.D., Editor, Natural Rubber Science and Technology, Malaysian Rubber Producers Research Association, 1988.
10. Blackly, D.C., Synthetic Rubbers: Their Chemistry and Technology, Chapter 2, Applied Science Publishers, London, 1983.
11. Sakdapipanich, J., Rojruthai, P., Molecular Structure of Natural Rubber and Its Characteristics Based on Recent Evidence, http://cdn.interopen.com/pdfs/29737/InTech-Molecular_structure_of_natural_rubber_and_its_characteristics_based_on_recent_evidence.pdf.
12. Tanaka, Yasuyuki, Structural Characterization of Natural Polyisoprenes: Solve the Mystery of Natural Rubber Based on Structural Study, Rubber Chemistry and Technology, Volume 74, Number 3.
13. Sakdapipanich, J., Rojruthai, P., Molecular Structure of Natural Rubber and Its Characteristics Based on Recent Evidence, http://cdn.interopen.com/pdfs/29737/InTech-Molecular_structure_of_natural_rubber_and_its_characteristics_based_on_recent_evidence.pdf.
14. Blackly, D.C., Synthetic Rubbers: Their Chemistry and Technology, Chapter 2, Applied Science Publishers, London, 1983.


